Batteries: Lithium-Ion Battery and Lithium Polymer Battery Explained
I’m sure everyone has definitely seen and used a battery before, you can find them literally everywhere! But do you know Lithium-Ion Batteries are a much more environmentally-friendly option than regular batteries?
As we all know, you won’t be able to reuse your regular batteries and they would always end up in the bin. But with a Lithium-Ion battery, you’ll be able to keep reusing your batteries, though it comes with a risk. Thus in this article, we will be talking about Lithium-Ion batteries and what you should take note of!
Before we get right into it, let’s recap a little about batteries.

Alkaline batteries or the regular batteries we refer to, contain chemicals that allow it to produce electricity. Alkaline batteries contain 3 chemicals inside the metal cell: zinc, manganese dioxide and potassium hydroxide. The brass pin in the middle allows the battery to conduct electricity to the circuit.
The structure of a regular battery look as such:
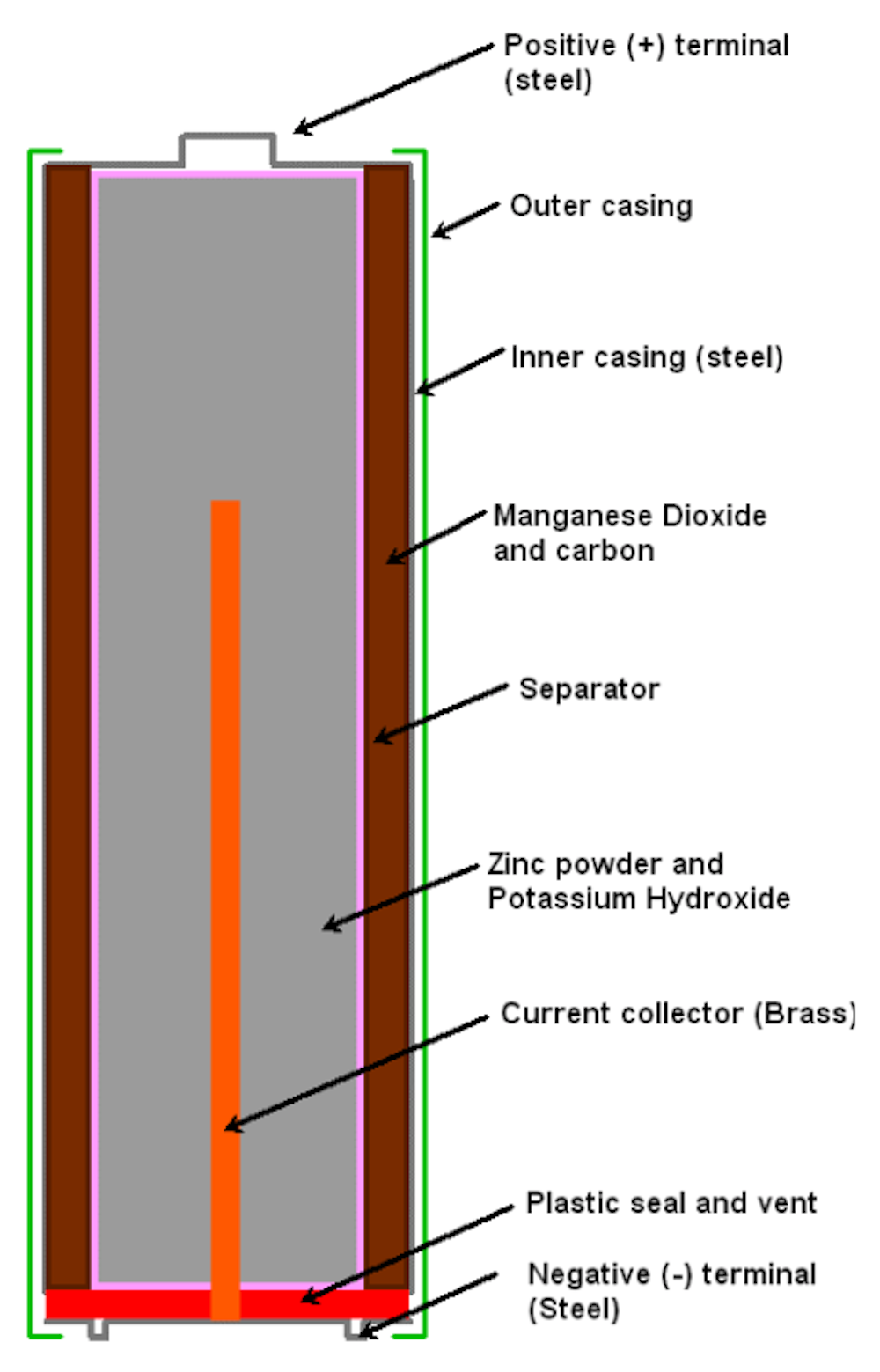
There is also a positive and negative terminal in each battery, which are connected to two separated metal plates respectively, known as electrodes. The chemicals will then react to the metal plates, where more electrons will be present at the negative terminal and fewer electrons will be present at the positive terminal. That difference in electrons will produce a voltage, which produces a constant flow of electrons from the negative terminal to the positive terminal.
If you’re interested to learn more about voltage, check out What happens in an electric circuit: Voltage vs Current!
With that said, let’s look at what will be covered today:
- Overview of Lithium-Ion Battery
- What’s special about Lithium polymer battery?
- Lithium Battery Products Recommendation
- Applications of Lithium-Ion Battery
- Projects with Lithium-Ion Battery
- Things to note when using a Lithium-Ion Battery
Overview of Lithium-Ion Battery
What is a Lithium-Ion Battery?
Lithium-Ion Batteries are rechargeable batteries, which uses lithium ions. It works the same way as a battery does, just an entirely different component (will address that later). There are also a few different types of Li-ion batteries available, but the most common one uses Lithium Cobalt and Graphite.

How does Lithium-Ion Battery work?
To understand how it works, let’s look at the basic structure of a common Li-ion battery:

- Cathode: Positive electrode (terminal), usually made out of lithium-ions.
- Electrolyte: Right between both cathode and anode (electrodes), typically a mixture of carbonates
- Anode: Negative electrode (terminal), usually made out of carbon (graphite).
- Separator: Made out of a thin sheet of microperforated plastic, preventing both electrodes from mixing with each other, but only allow the ions from interacting.
Thus, when the lithium battery is discharging, the anode will start oxidizing. This would cause the lithium ions to pass through the electrolyte and through the cathode, and other electrons to pass through the external circuit. These ions and electrons will then come together again at the cathode, this is also known as reduction reaction. In this way, the lithium battery would be able to store electrical energy.
Variants of Lithium-ion Batteries
We did mention that the most common Lithium-ion battery is made out of Lithium Cobalt and Graphite, but there are actually a few more others! They are quite similar to one another, but they have different uses. The main difference is in the material of anode and cathode.
Lithium Cobalt Oxide (LCO)

Like we mentioned, this particular lithium battery is what you might come across the most since you can find it in your phones, computers, tablets etc. This is actually one of the safer options and it offers high energy density. However, it has a short life-span and its current capacity is limited. Though nowadays manganese or nickel is added to help prolong its lifetime.
Lithium Manganese Oxide (LMO)
This lithium-ion battery uses lithium manganese oxide as the cathode which allows it to have a high current capability. However, it has a lower energy density and won’t last as long. You can find this type of lithium battery in power tools and medical tools etc.
Lithium Iron Phosphate Battery (LFP)
As the name suggests, it uses lithium iron phosphate as the cathode and provides a high current capability as well as a longer lifespan. Though this causes the LFP to discharge faster and to have a lower cell voltage when compared to its other counterparts. It can be found in power tools, medical tools too, similar to the previous LMO.
Lithium Nickel Manganese Cobalt Oxide (NMC)
This lithium battery has the most combination of metals, it consists of nickel, manganese and cobalt. But all of them are in equal parts, meaning 1:1:1. Though it’s popular to have 5 parts nickel, 2 parts manganese and 3 parts cobalt as well. This particular lithium battery has high specific energy but the energy density may not be as high. You can often find it in electric vehicles and power tools.
Lithium Nickel Cobalt Aluminium Oxide (NCA)
This type of lithium battery is less common, and it’s more so for the automotive industry. It features a high energy and power density, and last pretty long. However, it is relatively expensive and requires the user to be careful when using it.
Lithium vs Alkaline Batteries
Before we talk about another variant of Lithium batteries, here’s a comparison between Lithium and alkaline batteries:
Lithium-ion Batteries

Alkaline Batteries

Lithium Polymer Battery
Have you heard of another variant of Lithium battery? The Lithium Polymer battery or Li-po is generally quite similar to a Li-ion battery. Just that this technology is newer than Li-ion and similar to a Li-po, it has a positive and negative electrode but uses a gel-like electrolyte, instead of the liquid in Li-po.

Due to this change, Li-po is a tad safer than Li-ion as you wouldn’t risk leaking. Furthermore, it offers more versatility in design and a lower profile. But they last shorter and costs more than your Li-ion batteries.
Mini Comparison between Li-ion and Li-po batteries
| Lithium-Ion Battery | Lithium Polymer Battery |
| Cheap | Costly |
| High power density | Stores less power |
| Longer lifespan | Shorter lifespan |
| Higher profile | Low profile |
| Higher chance of electrolyte leakage | Lower chance of electrolyte leakage |
If you were to ask us, which is better? We can only say that Li-ion is more popular in consumer products due to its high power density, and it is cheaper too. But if you prioritise safety, and have a couple more coins to spare then definitely go for Li-po!
Lithium Battery Products Recommendation
Speaking of lithium battery, there is an item you’ll need to have in order to charge your battery. That’s right, a lithium battery charger!
LiPo Rider Pro ($14.95)

The LiPo Rider Pro is an upgraded version of our previous LiPo Rider ($9.50). As compared to the previous version, the upgraded LiPo Rider Pro has a higher load output and is able to run on solar power! Thus, it is very environmentally-friendly, easy to use and affordable as well.
Moreover, you can charge your LiPo batteries and charge your phone at the same time. It also features 2x USB ports that allow you to do programming and charge your battery at the same time.
Lipo Rider Plus (Charger/Booster) – 5V/2.4A USB Type C ($4.90)

Unlike our LiPo Rider Pro ($14.95), the Lipo Rider Plus won’t be able to charge using a solar panel. To make up for that, the Lipo Rider Plus features fast charging and a strong power boost for your microcontrollers and slave devices!
Moreover, due to its petite size, you can mount it on almost any projects you have in mind! All of this, at an affordable price tag of under $5! Making this pocket-friendly option for students.
Seeed also offers LiPo battery as well, so should you require one, click here for our Lithium-Ion polymer Battery pack – 6A!

Applications of Lithium-ion Battery
As we mentioned earlier, there’re so many electronics that use Lithium-ion batteries. We will give a few examples below:
Flashlight

Of course with anything, there are both alkaline and lithium versions of flashlights. But Lithium flashlights are a lot brighter and has more functions than an alkaline one. It also varies in sizes as well, not to mention how lithium flashlights would definitely run longer than an alkaline one!
Electric Cars

Though lithium batteries weren’t as popular due to how sensitive they were to temperature, which causes them to degrade really fast. However, after the modified lithium batteries were produced, car companies are beginning to implement it. Though it’ll still take time for all of the electric cars to use lithium batteries, we do see some uses of the lithium iron phosphate battery.
Projects with Lithium-ion Battery
Yapolamp

Are you looking to build a night lamp that would be easy on your eyes for reading? Look no further than this Yapolamp! With a name as cute as this, it is also safe for curious kids who want to play with lights constantly. This project will guide you through from the start till the end, so it’s perfect for beginners who’re looking for lithium battery projects!
What you’ll need:
- ATtiny85 (or RISC-Based Microcontrollers)
- Inductor (LED driver circuit component)
- Digital multimeter (optional)
- Oscilloscope (optional)
- Arduino Nano clone
- 300 Ohm Resistor
- 40 Ohm Resistor
- 3 x N channel MOSFET
- LEDs
- PCB
- Lithium-ion battery
- Battery charge controller
- Pull-down resistor for MOSFET gate
If this sounds like a project you’re interested in, click here to find out more!
Tetris Light – Battery Mod

Interested in hacking to fit in a Lithium battery and USB charger? This project takes Tetris light as an example to modify the battery which would give it more mobility. If you’re new to hacking, you can take this project as a reference!
What you’ll need (Using Tetris light as an example):
- Tetris Light
- Lithium-ion battery
- Lithium-ion battery charger
- Lithium-ion battery holder
- 5V booster convertor
- Cables
- Hot glue gun
Did this project spark your interest? Feel free to click here and start hacking!
Things to note when using a Lithium-Ion Battery
Though Lithium-ion battery comes with a lot of advantages, there are some precautions we need to take when using it as it can cause a fire hazard. I’m sure nobody would want to get hurt when trying to incorporate lithium battery in their projects!
- Do partial discharge, don’t totally discharge: Lithium batteries do not have a ‘memory’, so partial discharge is alright. But leaving them totally discharge can shorten their lifespan.
- Do keep cool, don’t freeze: Yes we get it, it can be pretty tempting to help cool down your lithium battery faster when it’s heating up, especially not in the freezer. Most lithium-ion battery electrolytes freeze around -40°C, but you can store it in your refrigerator to slow down its ageing process.
- Do buy when you need them, don’t let it sit on your shelf: Lithium batteries only last 2~3 years, and they would start ageing the moment they’re manufactured. Storing your lithium batteries instead of using them will only let your money go to waste.
Summary
And that’s all on Lithium-ion batteries! Did you learn something new? It may sound daunting due to the precautions, but as long as you don’t allow your lithium batteries to overheat, you’ll be fine! Hope that you enjoyed this article, if you’re interested in articles as such, do check out the related readings down below!
Suggested Readings
All about CPUs: Microprocessor, Microcontroller and Single Board Computer – Interested in CPU contents? Check this article out!
What causes laptop batteries to overheat? – To learn more about how do batteries overheat!
Electronics Circuit: Voltage Dividers – Want to prevent fire hazards? A Voltage divider will be your best friend!